IB Diploma Chemistry
Chemistry is an experimental science that combines academic study with the acquisition of practical and investigational skills. It is often called the central science, as chemical principles underpin both the physical environment in which we live and all biological systems. Apart from being a subject worthy of study in its own right, chemistry is a prerequisite for many other courses in higher education, such as medicine, biological science and environmental science, and serves as useful preparation for employment. The Diploma Programme chemistry course includes the essential principles of the subject but also, through selection of an option, allows teachers some flexibility to tailor the course to meet the needs of their students. The course is available at both standard level (SL) and higher level (HL), and therefore accommodates students who wish to study chemistry as their major subject in higher education and those who do not.
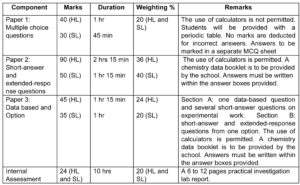
Source: IBDP Chemistry Guide 2016
MODULES : View - Register - Learn
If you are new to IBDP or coming from a non-IB curriculum, the students often get lost and confused as to what’s the programme all about as the school sets in. It creates stress of the overwhelming number of tasks and assignments that schools give as soon as the DP programme begins. This module shall equip students with the knowledge of structure and framework of the Chemistry subject as per IB standards and expectations. This module shall equip students with the bridge to smoothly have a transition from their previous learning, irrespective of the board/curriculum that they studied as to have a kind of immersion programme to naturally transit into the IBDP programme. The module explores the subject guide to understand the various aspects of DP programme, like the command terms, objectives, nature of science, TOK links, theory topics, components, differences between SL and HL and certain other key aspects of DP programme.
The Assessment pattern of the subject shall be elaborated with an activity wherein students shall get an opportunity to see IB assessment types, ideas to solve questions and have an overview of the most challenging part of the programme which is Internal assessment weighing 20%.
This module shall specifically look into the chemistry language, terminology, scientific terms and focus on the skills necessary to score high in the board exams. Focus on chemical formulae, balancing equations, types of particles, valency, radicals, atomic structure and periodic table basics, etc shall be covered.
Duration of the Module: 4 Hours (Two sessions of 2 hours each)
Module CODE: IBCHEM1
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The arrangement of elements in the periodic table helps to predict their electron configuration. Elements show trends in their physical and chemical properties across periods and down groups. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (three sessions of 2 hours each)
Module CODE: IBCHEM2
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The transition elements have characteristic properties; these properties are related to their all having incomplete d sublevels. d-orbitals have the same energy in an isolated atom, but split into two sub-levels in a complex ion. The electric field of ligands may cause the d-orbitals in complex ions to split so that the energy of an electron transition between them corresponds to a photon of visible light. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (Two sessions of 2 hours each)
Module CODE: IBCHEM3
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The mass of an atom is concentrated in its minute, positively charged nucleus. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (two sessions of 2 hours each)
Module CODE: IBCHEM4
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The quantized nature of energy transitions is related to the energy states of electrons in atoms and molecules. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 2 Hours (One session)
Module CODE: IBCHEM5
Fee: SGD 80/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Ionic compounds consist of ions held together in lattice structures by ionic bonds. Covalent compounds form by the sharing of electrons. Lewis (electron dot) structures show the electron domains in the valence shell and are used to predict molecular shape. The physical properties of molecular substances result from different types of forces between their molecules. Metallic bonds involve a lattice of cations with delocalized electrons. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 10 Hours (Five sessions of 2 hours each)
Module CODE: IBCHEM6
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Larger structures and more in-depth explanations of bonding systems often require more sophisticated concepts and theories of bonding. Hybridization results from the mixing of atomic orbitals to form the same number of new equivalent hybrid orbitals that can have the same mean energy as the contributing atomic orbitals. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM7
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Physical and chemical properties depend on the ways in which different atoms combine. The mole makes it possible to correlate the number of particles with the mass that can be measured. Mole ratios in chemical equations can be used to calculate reacting ratios by mass and gas volume. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 10 (Five sessions of 2 hours each)
Module CODE: IBCHEM8
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The enthalpy changes from chemical reactions can be calculated from their effect on the temperature of their surroundings. In chemical transformations energy can neither be created nor destroyed (the first law of thermodynamics). Energy is absorbed when bonds are broken and is released when bonds are formed. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM9
Fee: SGD 320/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The concept of the energy change in a single step reaction being equivalent to the summation of smaller steps can be applied to changes involving ionic compounds. A reaction is spontaneous if the overall transformation leads to an increase in total entropy (system plus surroundings). The direction of spontaneous change always increases the total entropy of the universe at the expense of energy available to do useful work. This is known as the second law of thermodynamics. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM10
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The greater the probability that molecules will collide with sufficient energy and proper orientation, the higher the rate of reaction. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (Two sessions of 2 hours each)
Module CODE: IBCHEM11
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Rate expressions can only be determined empirically and these limit possible reaction mechanisms. In particular cases, such as a linear chain of elementary reactions, no equilibria and only one significant activation barrier, the rate equation is equivalent to the slowest step of the reaction. The activation energy of a reaction can be determined from the effect of temperature on reaction rate. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM12
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Many reactions are reversible. These reactions will reach a state of equilibrium when the rates of the forward and reverse reaction are equal. The position of equilibrium can be controlled by changing the conditions. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (Two sessions of 2 hours each)
Module CODE: IBCHEM13
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The position of equilibrium can be quantified by the equilibrium law. The equilibrium constant for a particular reaction only depends on the temperature. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (two sessions of 2 hours each)
Module CODE: IBCHEM14
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Many reactions involve the transfer of a proton from an acid to a base. The characterization of an acid depends on empirical evidence such as the production of gases in reactions with metals, the colour changes of indicators or the release of heat in reactions with metal oxides and hydroxides. The pH scale is an artificial scale used to distinguish between acid, neutral and basic/alkaline solutions. The pH depends on the concentration of the solution. The strength of acids or bases depends on the extent to which they dissociate in aqueous solution. Increased industrialization has led to greater production of nitrogen and sulfur oxides leading to acid rain, which is damaging our environment. These problems can be reduced through collaboration with national and intergovernmental organizations. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM15
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: The acid–base concept can be extended to reactions that do not involve proton transfer. The equilibrium law can be applied to acid–base reactions. Numerical problems can be simplified by making assumptions about the relative concentrations of the species involved. The use of logarithms is also significant here. pH curves can be investigated experimentally but are mathematically determined by the dissociation constants of the acid and base. An indicator with an appropriate endpoint can be used to determine the equivalence point of the reaction. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM16
Fee: SGD 320/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Redox (reduction–oxidation) reactions play a key role in many chemical and biochemical processes. Voltaic cells convert chemical energy to electrical energy and electrolytic cells convert electrical energy to chemical energy. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM17
Fee: SGD 320/
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Energy conversions between electrical and chemical energy lie at the core of electrochemical cells. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM18
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Organic chemistry focuses on the chemistry of compounds containing carbon. Structure, bonding and chemical reactions involving functional group interconversions are key strands in organic chemistry. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 10 Hours (Five sessions of 2 hours each)
Module CODE: IBCHEM19
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Key organic reaction types include nucleophilic substitution, electrophilic addition, electrophilic substitution and redox reactions. Reaction mechanisms vary and help in understanding the different types of reaction taking place. Organic synthesis is the systematic preparation of a compound from a widely available starting material or the synthesis of a compound via a synthetic route that often can involve a series of different steps. Stereoisomerism involves isomers which have different arrangements of atoms in space but do not differ in connectivity or bond multiplicity (i.e. whether single, double or triple) between the isomers themselves. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 10 Hours (Five sessions of 2 hours each)
Module CODE: IBCHEM20
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: All measurement has a limit of precision and accuracy, and this must be taken into account when evaluating experimental results. Graphs are a visual representation of trends in data. Analytical techniques can be used to determine the structure of a compound, analyse the composition of a substance or determine the purity of a compound. Spectroscopic techniques are used in the structural identification of organic and inorganic compounds. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 10 Hours (Five sessions of 2 hours each)
Module CODE: IBCHEM21
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the essential ideas such as: Although spectroscopic characterization techniques form the backbone of structural identification of compounds, typically no one technique results in a full structural identification of a molecule. The students will be able to apply this learning and skills needed for paper 1, 2 and 3 in the exam.
Duration of the Module: 4 Hours (Two sessions of 2 hours each)
Module CODE: IBCHEM22
Fee: SGD 160/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the following essential key concepts. They will be able to apply this learning and skills required for paper 3 in the exam.
A.1 Materials science introduction
A.2 Metals and inductively coupled plasma (ICP) spectroscopy
A.3 Catalysts
A.4 Liquid crystals
A.5 Polymers
A.6 Nanotechnology
A.7 Environmental impact—plastics
Duration of the Module: 12 Hours (Six sessions of 2 hours each)
Module CODE: IBCHEM23
Fee: SGD 480/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the following essential key concepts. They will be able to apply this learning and skills required for paper 3 in the exam.
A.8 Superconducting metals and X-ray crystallography
A.9 Condensation polymers
A.10 Environmental impact—heavy metals
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM24
Fee: SGD 320/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the following essential key concepts. They will be able to apply this learning and skills required for paper 3 in the exam.
C.1 Energy sources
C.2 Fossil fuels
C.3 Nuclear fusion and fission
C.4 Solar energy
C.5 Environmental impact—global warming
Duration of the Module: 12 Hours (six sessions of 2 hours each)
Module CODE: IBCHEM25
Fee: SGD 480/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, student shall learn the following essential key concepts. They will be able to apply this learning and skills required for paper 3 in the exam.
C.6 Electrochemistry, rechargeable batteries and fuel cells
C.7 Nuclear fusion and nuclear fission
C.8 Photovoltaic and dye-sensitized solar cells
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM26
Fee: 320/- SGD
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module students shall be provided with the essential skills to do problem solving, scientific reasoning and structure their answers as needed by IB assessment standards. Tips, tricks and techniques with respect to MCQ, structured answers, extended response, data based questions, data analysis and evaluation, using scientific terms to have focused answers, avoiding pitfalls and traps, etc shall be shared. IB Mark Schemes and examiner remarks shall be explained.
Duration of the Module: 8 Hours (Four sessions of 2 hours each)
Module CODE: IBCHEM27
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, students shall be given training in Mandatory practicals which shall help solving paper 3 section A. The students will be able to apply this knowledge and skills needed for paper 3 section A in the exam.
Duration of the Module: 10 Hours (scheduled as required)
Module CODE: IBCHEM28
Fee: SGD 400/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
In this module, the students learn the essential skills of the following. The students will be able to apply the knowledge and skills needed for Internal assessment which weighs overall 20% in the subject.
- Marking criteria and rubrics
- Lab Report template
- Checklist
- IA Resources
Duration of the Module: 6 Hours (Three sessions of 2 hours each)
Module CODE: IBCHEM29
Fee: SGD 240/-
Mode of Teaching: Online
How to enrol: Fill in the form and submit for slots and payment details CLICK HERE
